Lumpy skin disease
Lumpy skin disease (LSD) is caused by lumpy skin disease virus (LSDV), a virus from the Poxviridae family. LSDV belongs, together with sheeppox virus (SPPV) and goatpox virus (GTPV), to the genus of Capripoxviruses.
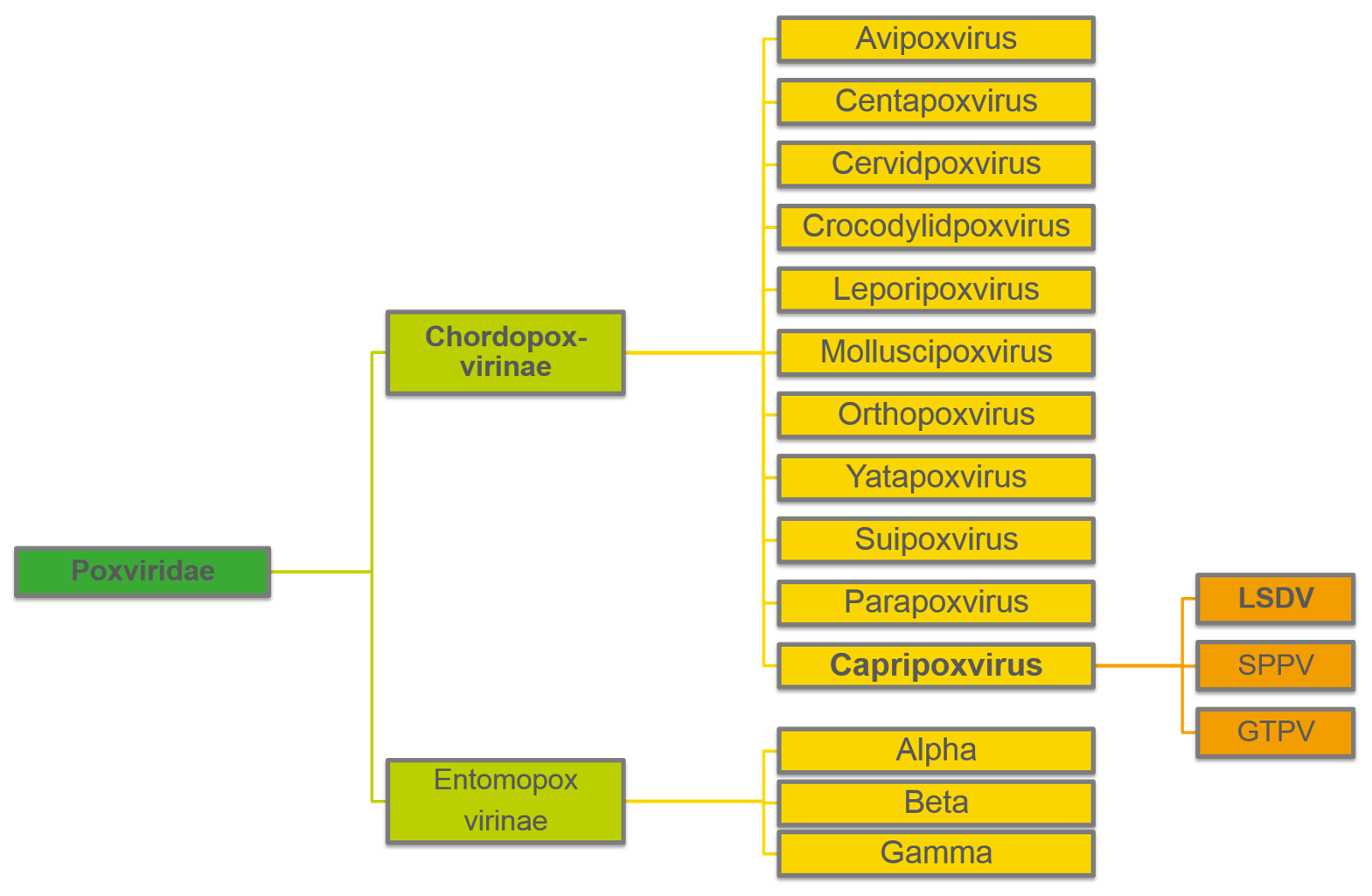
LSDV is highly host specific causing disease in cattle (Bos indicus and B. taurus) and water buffalo (Bubalus bubalis). There is no wildlife reservoir of LSDV and LSDV is not zoonotic.

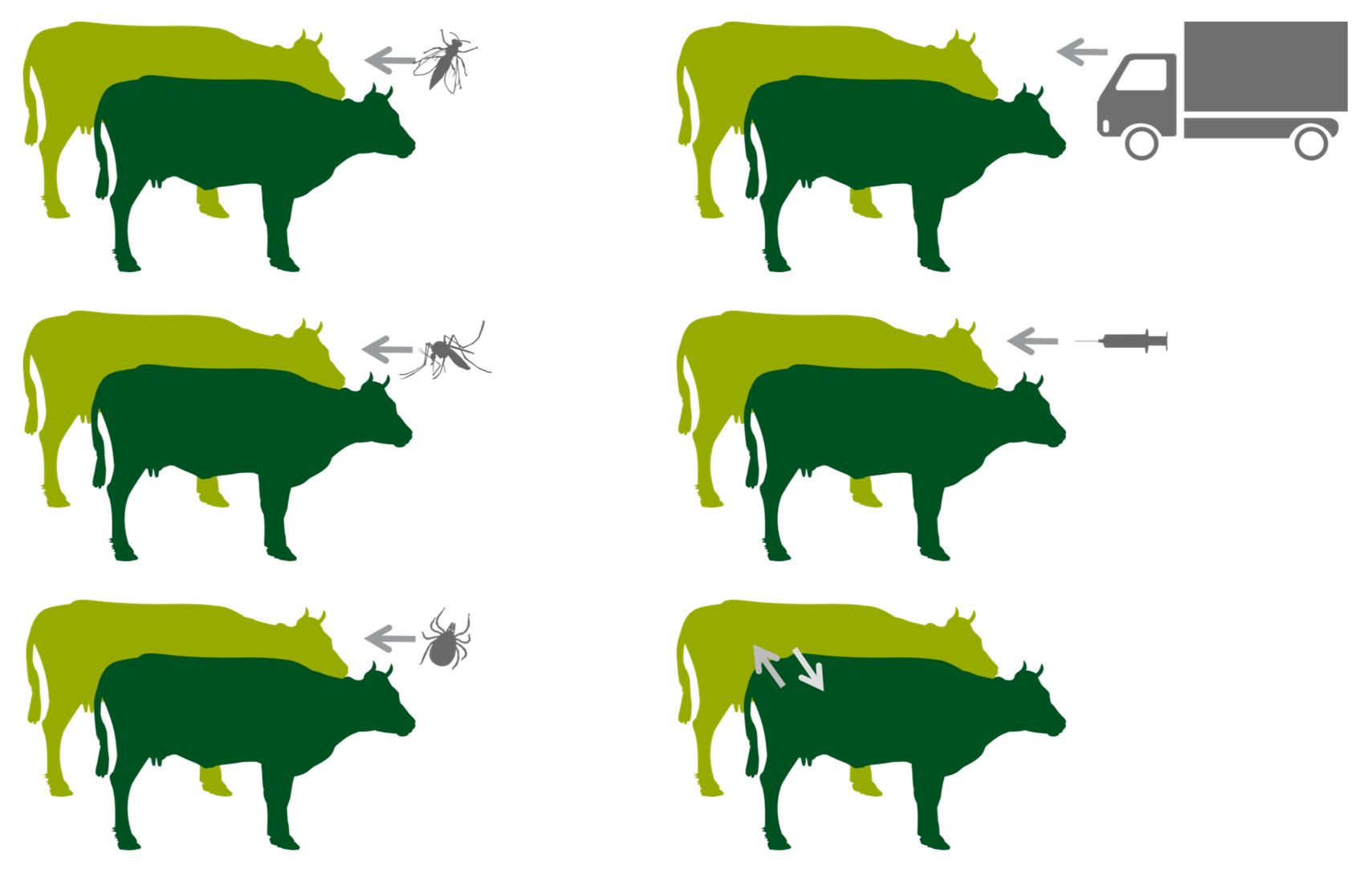
other modes of transmission (right) are considered to play minor roles
Transmission is believed to occur mainly by arthropod vector. No specific vector has been identified so far, certain species of mosquitoes, biting flies and male ticks could play a role in the transmission of the virus. The importance of each specific arthropod vectors may vary in different areas depending on the abundance and feeding behaviour of the vector. It is not known if transmission can occur via fomites. Direct contact is considered to play a minor role in the transmission of LSDV. Infected bulls can excrete the virus in the semen, although transmission of LSD via infected semen has not been demonstrated yet.
LSD signs range from subclinical to severe disease. Symptoms include fever, nodules on the skin, mucous membranes and internal organs, emaciation, enlarged lymph nodes, oedema of the skin, and sometimes death. There is no current evidence of variation in virulence of the different LSDV strains.

The disease is of economic importance as it can cause a temporary reduction in milk production, infertility, temporary or permanent sterility in bulls, damage to hides and death due to secondary bacterial infections. Skin nodules, scabs and crusts contain relatively high amounts of LSDV. The virus is very stable in these materials and can be isolated for at least 35 days. LSDV can also be isolated from blood, saliva, ocular and nasal discharge, and semen. LSDV is found in the blood from approximately 7 to 21 days post-infection, but at lower levels than in skin nodules. LSD does not cause chronic disease. It does not exhibit latency and recrudescence of disease does not occur.
LSD is categorized on the list of notifiable diseases by the World Organization for Animal Health (WOAH) due to its ability of rapid transboundary spread and because of important cattle production losses. LSD is endemic in most African countries. Since 2012 it has spread rapidly through the Middle East, southeast Europe, the Balkans, Caucasus, Russia and Kazakhstan.
In 2017, there was the emergence of new strains in Russia. Vaccine-like strains of LSDV were found in outbreaks close to the border with Kazakhstan. However no homologues vaccination was used is Russia. But Kevevapi, a homologous vaccine was used in Kazakhstan. Although marketed as a Neethling (clade 1.1) vaccine, different LSD vaccine strains and even a GTPV strain were found in this vaccine during a first analysis. Whole genome sequencing in 2022 revealed that the vaccine contained indeed the three earlier mentioned strains, as well as several recombinant strains, containing LSDV Neethling and KSGP vaccine sequences. In the meantime, different outbreaks were seen in South-East Asian with strains similar to the strain detected in Russia. The main difference between these new strains and the older strains is the mode of transmission. While direct and indirect transmission plays only a minor role in the "classical" strains, it seems to play a way more important role with the new strains.
For the most recent, detailed information on the occurrence of this disease worldwide, see the World Animal Health Information System (WAHIS) Interface.
Laboratory confirmation of LSD is most rapid using a real-time or conventional polymerase chain reaction (PCR) method specific for capripoxviruses in combination with a clinical history of a generalised nodular skin disease and enlarged superficial lymph nodes in cattle. LSDV will grow in tissue culture of bovine, ovine or caprine origin, particularly using lamb testis or bovine dermis cells. Capripoxvirus antigens can be demonstrated in tissue culture using immunoperoxidase or immunofluorescent staining.The virus neutralisation test (VNT) is the only validated serological test available. Western blotting using the reaction between the P32 antigen of LSDV with test sera is both sensitive and specific, but is difficult and expensive to carry out. Some antibody-detecting enzyme-linked immunosorbent assays (ELISAs) have been described and have been released on to the market.
Outbreaks of LSD revealed that successful control and eradication of LSD relies on early detection of the index case, followed by a rapid and widespread vaccination campaign (see video below). Attenuated cattle strains, and strains derived from sheep and goats have been used as live vaccines against LSDV. Total or partial stamping-out policies have also been used to eradicate the infection.
Sanitary prophylaxis in free countries include import restrictions on domestic cattle and water buffaloes, and selected products from these animals as well as surveillance measures to detect LSD over a distance of at least 20 kilometres from an infected country or zone.
In infected countries control of LSD depends on restriction of movement of cattle in infected regions, removal of clinically affected animals, and vaccination. Movement restrictions and removal of affected animals alone without vaccination are usually not effective. Proper disposal of dead animals
(e.g. incineration), and cleaning and disinfection of premises and implements are recommended for LSD. There is currently no evidence of the efficacy of vector control in preventing disease
Medical prophylaxis include “Homologous” LSDV live attenuated vaccine strain for example “Neethling” LSD strain, “Heterologous” sheeppox or goatpox virus live attenuated vaccine strain. A local reaction at the site of inoculation, as well as fever and reduction in milk yield, may follow vaccination
with live, attenuated capripox virus. Currently, no new generation recombinant capripox vaccines are commercially available.
For more detailed information regarding LSD refer to Chapter 3.4.121 Lumpy skin disease in the latest edition of the WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals
Version 10.0
